What Is the Minimum Energy Required to Ionize a Hydrogen Atom in the N=3

Ionization energy trends plotted against the diminutive number, in units eV. The ionization energy gradually increases from the alkali metals to the noble gases. The maximum ionization energy also decreases from the first to the final row in a given column, due to the increasing distance of the valence electron shell from the nucleus. Predicted values are used for elements beyond 104.
In physics and chemical science, ionization energy (IE) (American English language spelling), ionisation energy (British English language spelling) is the minimum free energy required to remove the most loosely bound electron of an isolated neutral gaseous atom or molecule.[ane] Information technology is quantitatively expressed as
- Ten(g) + energy ⟶ X+(g) + e−
where Ten is whatever atom or molecule, X+ is the resultant ion when the original atom was stripped of a single electron, and e− is the removed electron.[ii] Ionization energy is positive for neutral atoms, meaning that the ionization is an endothermic process. Roughly speaking, the closer the outermost electrons are to the nucleus of the cantlet, the higher the atom'south ionization free energy.
In physics, ionization energy is usually expressed in electronvolts (eV) or joules (J). In chemical science, it is expressed every bit the energy to ionize a mole of atoms or molecules, usually every bit kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol).[three]
Comparison of ionization energies of atoms in the periodic tabular array reveals two periodic trends which follow the rules of Coulombic attraction:[4]
- Ionization energy generally increases from left to correct within a given menses (that is, row).
- Ionization free energy generally decreases from elevation to lesser in a given grouping (that is, cavalcade).
The latter tendency results from the outer electron shell existence progressively further from the nucleus, with the add-on of one inner shell per row every bit 1 moves down the column.
The nthursday ionization free energy refers to the corporeality of energy required to remove the about loosely jump electron from the species having a charge of (n − 1). For example, the start 3 ionization energies are defined as follows:
- 1st ionization energy is the free energy that enables the reaction X ⟶ X+ + e−
- 2nd ionization free energy is the energy that enables the reaction 10+ ⟶ X2+ + e−
- tertiary ionization free energy is the energy that enables the reaction Xtwo+ ⟶ Ten3+ + e−
The most notable influences that determine ionization energy include:
- Electron configuration: This accounts for most element's IE, as all of their chemical and concrete characteristics can be ascertained just by determining their corresponding electron configuration.
- Nuclear charge: If the nuclear accuse (diminutive number) is greater, the electrons are held more tightly by the nucleus and hence the ionization energy will be greater.
- Number of electron shells: If the size of the cantlet is greater due to the presence of more shells, the electrons are held less tightly by the nucleus and the ionization energy will be lesser.
- Constructive nuclear charge (Z eff): If the magnitude of electron shielding and penetration are greater, the electrons are held less tightly past the nucleus, the Z eff of the electron and the ionization energy is lesser.[five]
- Stability: An atom having a more than stable electronic configuration has less tendency to lose electrons and consequently has higher ionization energy.
Pocket-size influences include:
- Relativistic Effects: Heavier elements (specially those whose atomic number is greater than nigh 70) are affected past these every bit their electrons are approaching the speed of low-cal. They therefore take smaller atomic radii and higher ionization energies.
- Lanthanide And Actinide contraction (and scandide wrinkle): The unprecedented shrinking of the elements bear upon the ionization energy, as the net charge of the nucleus is more than strongly felt.
- Electron pairing energies: Half-filled subshells usually result in higher ionization energies.
The term ionization potential is an older and obsolete term[half dozen] for ionization energy,[7] because the oldest method of measuring ionization energy was based on ionizing a sample and accelerating the electron removed using an electrostatic potential.
Determination of ionization energies [edit]

Ionization free energy measurement apparatus.
Ionization energy of atoms, denoted E i, is measured[viii] by finding the minimal energy of light quanta (photons) or electrons accelerated to a known free energy that will kick out the least bound diminutive electrons. The measurement is performed in the gas phase on single atoms. While just noble gases occur as monatomic gases, other gases can be split into single atoms.[9] Too, many solid elements tin be heated and vaporized into unmarried atoms. Monatomic vapor is contained in a previously evacuated tube that has two parallel electrodes connected to a voltage source. The ionizing excitation is introduced through the walls of the tube or produced inside.
When ultraviolet light is used, the wavelength is swept downward the ultraviolet range. At a sure wavelength (λ) and frequency of light (ν=c/λ, where c is the speed of light), the light quanta, whose energy is proportional to the frequency, volition take energy loftier enough to dislodge the least spring electrons. These electrons will be attracted to the positive electrode, and the positive ions remaining subsequently the photoionization will get attracted to the negatively charged electrode. These electrons and ions will establish a current through the tube. The ionization energy will exist the energy of photons hν i (h is the Planck abiding) that acquired a steep rise in the electric current: E i=hν i.
When high-velocity electrons are used to ionize the atoms, they are produced by an electron gun inside a similar evacuated tube. The energy of the electron beam can be controlled past the dispatch voltages. The free energy of these electrons that gives rise to a precipitous onset of the electric current of ions and freed electrons through the tube will match the ionization free energy of the atoms.
Atoms: values and trends [edit]
Generally, the (N+1)th ionization energy of a detail element is larger than the Nth ionization free energy(too it may be noted that ionization energy of an anion is generally less than that of cations and neutral atom for same element). When the next ionization free energy involves removing an electron from the same electron shell, the increase in ionization energy is primarily due to the increased net charge of the ion from which the electron is being removed. Electrons removed from more than highly charged ions experience greater forces of electrostatic allure; thus, their removal requires more energy. In addition, when the next ionization energy involves removing an electron from a lower electron shell, the greatly decreased altitude between the nucleus and the electron also increases both the electrostatic strength and the altitude over which that forcefulness must exist overcome to remove the electron. Both of these factors further increase the ionization free energy.
Some values for elements of the third period are given in the post-obit table:
| Element | First | 2nd | Third | Quaternary | Fifth | 6th | Seventh |
|---|---|---|---|---|---|---|---|
| Na | 496 | 4,560 | |||||
| Mg | 738 | i,450 | 7,730 | ||||
| Al | 577 | ane,816 | 2,881 | xi,600 | |||
| Si | 786 | i,577 | 3,228 | 4,354 | 16,100 | ||
| P | ane,060 | 1,890 | two,905 | 4,950 | 6,270 | 21,200 | |
| S | 1,000 | 2,295 | iii,375 | iv,565 | 6,950 | 8,490 | 27,107 |
| Cl | 1,256 | 2,260 | 3,850 | v,160 | 6,560 | 9,360 | eleven,000 |
| Ar | 1,520 | 2,665 | 3,945 | five,770 | 7,230 | viii,780 | 12,000 |
Large jumps in the successive tooth ionization energies occur when passing element of group 0 configurations. For instance, as can exist seen in the table above, the first two molar ionization energies of magnesium (stripping the 2 3s electrons from a magnesium atom) are much smaller than the third, which requires stripping off a 2p electron from the neon configuration of Mgii+. That electron is much closer to the nucleus than the 3s electron removed previously.

Ionization energies peak in noble gases at the end of each period in the periodic table of elements and, equally a rule, dip when a new shell is starting to fill.
Ionization energy is also a periodic tendency within the periodic tabular array. Moving left to right within a period, or upward within a group, the start ionization energy generally increases,[10] with exceptions such as aluminium and sulfur in the tabular array in a higher place. As the nuclear accuse of the nucleus increases across the period, the electrostatic attraction increases between electrons and proton, hence the atomic radius decreases, and the electron deject becomes closer towards the nucleus[11] considering the electrons, particularly the outermost one, are held tighter by the higher effective nuclear charge.
On moving downward within a given group, the electrons are held in higher-energy shells with higher main quantum number n, further from the nucleus and therefore are more than loosely bound so that the ionization free energy decreases. The constructive nuclear charge increases but slowly and so that its upshot is outweighed by the increase in north.[12]
Exceptions in ionization energies [edit]
There are exceptions to the general trend of ascension ionization energies inside a period. For example, the value decreases from beryllium (
4 Be
: 9.3 eV) to boron (
five B
: 8.3 eV), and from nitrogen (
7 N
: 14.v eV) to oxygen (
viii O
: thirteen.half dozen eV). These dips can be explained in terms of electron configurations.[13]

The added electron in boron occupies a p-orbital.
Boron has its last electron in a 2p orbital, which has its electron density further away from the nucleus on average than the 2s electrons in the same shell. The 2s electrons then shield the 2p electron from the nucleus to some extent, and it is easier to remove the 2p electron from boron than to remove a 2s electron from beryllium, resulting in lower ionization energy for B.[2]

These electron configurations do not show the full and one-half-filled orbitals.
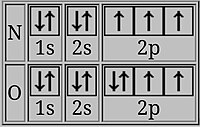
Here the added electron has a spin opposed to the other 2p electrons. This decreases the ionization energy of oxygen
In oxygen, the last electron shares a doubly occupied p-orbital with an electron of opposing spin. The 2 electrons in the same orbital are closer together on average than two electrons in different orbitals, so that they shield each other more finer and it is easier to remove one, resulting in lower ionization free energy.[2] [xiv]
Furthermore, after every noble gas element, the ionization energy drastically drops. This occurs because the outer electron in the alkali metals requires a much lower amount of energy to be removed from the atom than the inner shells. This too gives rising to depression electronegativity values for the brine metals.[xv] [16] [17]

Because of a unmarried p-orbital electron in gallium's configuration, makes the overall structure less stable, hence the dip in ionization free energy values[five]

Actinium's electron configuration predetermines that information technology would require less energy in society to remove that single d-orbital electron, ergo even though it has a larger EC, radium still has the higher IE[18]
The trends and exceptions are summarized in the following subsections:
Ionization energy decreases when [edit]
- Transitioning to a new flow: an element of group i easily loses i electron to leave an octet or pseudo-noble gas configuration, so those elements have only small values for IE.
- Moving from the s-block to the p-block: a p-orbital loses an electron more easily. An case is beryllium to boron, with electron configuration 1s2 2stwo 2p1. The 2s electrons shield the college-energy 2p electron from the nucleus, making it slightly easier to remove. This also happens in magnesium to aluminum.[19]
- Occupying a p-subshell with its offset electron with spin opposed to the other electrons: such equally in nitrogen (
vii N
: 14.v eV) to oxygen (
8 O
: thirteen.half-dozen eV), also as phosphorus (
15 P
: 10.48 eV) to sulfur (
sixteen S
: 10.36 eV). The reason for this is because oxygen, sulfur and selenium all take dipping ionization energies because of shielding effects.[20] Nevertheless, this discontinues starting from tellurium where the shielding is too small to produce a dip. - Moving from the d-block to the p-block: as in the case of zinc (
thirty Zn
: nine.4 eV) to gallium (
31 Ga
: half dozen.0 eV) - Special case: decrease from pb (
82 Lead
: 7.42 eV) to bismuth (
83 Bi
: 7.29 eV). This cannot be attributed to size (the difference is minimal: lead has a covalent radius of 146 pm whereas bismuth'due south is 148 pm[21]). Nor can information technology be attributed to relativistic stabilization of the 6s orbital, as this factor is very similar in the ii adjacent elements. Other factors suggest contrary to fact that bismuth should accept the higher IE, due to its half-filled orbital (calculation stabilization), position in the periodic table (Bi is farther right so it should exist less metallic than Pb), and it has ane more than proton (contributes to the [constructive] nuclear accuse).[22] - Special instance: decrease from radium (
88 Ra
: v.27 eV) to actinium (
89 Ac
: 5.17 eV) which is a switch from an s to a d orbital. However the analogous switch from barium (
56 Ba
: 5.two eV) to lanthanum (
57 La
: v.6 eV) does not show a down change. - Lutetium (
71 Lu
) and lawrencium (
103 Lr
) both have ionization energies lower than the previous elements. In both cases the last electron added starts a new subshell: 5d for Lu with electron configuration [Xe] 4ffourteen 5d1 6s2, and 7p for Lr with configuration [Rn] 5f4 7s2 7p1. These dips in ionization energies have since been used equally evidence in the ongoing debate over whether Lu and Lr should be placed in Grouping 3 of the periodic table instead of lanthanum (La) and actinium (Ac).[23] [24] [25] [26]
Ionization free energy increases when [edit]
- Reaching Grouping xviii noble gas elements: This is due to their consummate electron subshells,[27] so that these elements require big amounts of energy to remove ane electron.
- Grouping 12: The elements here, zinc (
30 Zn
: 9.iv eV), cadmium (
48 Cd
: ix.0 eV) and mercury (
80 Hg
: 10.4 eV) all tape sudden rising IE values in contrast to their preceding elements: copper (
29 Cu
: 7.7 eV), silvery (
47 Ag
: 7.6 eV) and gold (
79 Au
: 9.ii eV) respectively. For mercury, it can be extrapolated that the relativistic stabilization of the 6s electrons increases the ionization free energy, in improver to poor shielding by 4f electrons that increases the effective nuclear accuse on the outer valence electrons. In add-on, the closed-subshells electron configurations: [Ar] 3d10 4s2, [Kr] 4d105s2 and [Xe] 4f14 5d10 6stwo provide increased stability. - Special example: shift from rhodium (
45 Rh
: 7.five eV) to palladium (
46 Pd
: 8.three eV). Dissimilar other Group 10 elements, palladium has a college ionization energy than the preceding atom, due to its electron configuration. In contrast to nickel'south [Ar] 3d8 4s2, and platinum's [Xe] 4f14 5d9 6s1, palladium's electron configuration is [Kr] 4d10 5s0 (fifty-fifty though the Madelung rule predicts [Kr] 4d8 5s2). Finally, silverish'south lower IE (
47 Ag
: 7.six eV) further accentuates the high value for palladium; the single added southward electron is removed with a lower ionization free energy than palladium,[28] which emphasizes palladium's loftier IE (as shown in the above linear tabular array values for IE) - The IE of gadolinium (
64 Gd
: half dozen.fifteen eV) is somewhat higher than both the preceding (
62 Sm
: 5.64 eV), (
63 Eu
: five.67 eV) and following elements (
65 Tb
: 5.86 eV), (
66 Dy
: v.94 eV). This anomaly is due to the fact that gadolinium valence d-subshell borrows 1 electron from the valence f-subshell. Now the valence subshell is the d-subshell, and due to the poor shielding of +ve nuclear charge by electrons of the f-subshell, the electron of the valence d-subshell experiences a greater attraction to the nucleus, therefore increasing the energy required to remove the (outermost) valence electron. - Moving into d-block elements: The elements Sc with a 3done electronic configuration has a higher IP (
21 Sc
: 6.56 eV) than the preceding chemical element (
20 Ca
: 6.eleven eV), contrary to the decreases on moving into s-block and p-block elements. The 4s and 3d electrons have similar shielding power: the 3d orbital forms function of the n=3 shell whose average position is closer to the nucleus than the 4s orbital and the due north=4 trounce, but electrons in s orbitals experience greater penetration into the nucleus than electrons in d orbitals. And then the common shielding of 3d and 4s electrons is weak, and the effective nuclear accuse interim on the ionized electron is relatively large. Yttrium (
39 Y
) similarly has a college IP (half-dozen.22 eV) than
38 Sr
: 5.69 eV. The last ii d1 elements (
57 La
: 5.eighteen eV) and (
89 Ac
: 5.17 eV) have only very slightly lower IP's than their preceding elements (
56 Ba
: v.21 eV) and (
88 Ra
: 5.18 eV). - Moving into f-block elements; Every bit tin can be seen in the above graph for ionization energies, the sharp rise in IE values from (
55 Cs
) to (
57 La
) is followed by a small almost linear increase equally f electrons are added. This is due to the lanthanide contraction (for lanthanides).[29] [thirty] [31] This decrease in ionic radius is associated with an increase in ionization energy in plough increases, since the two properties correlate to each other.[ten] Every bit for d-cake elements, the electrons are added in an inner trounce, so that no new shells are formed. The shape of the added orbitals prevents them from penetrating to the nucleus so that the electrons occupying them take less shielding capacity.
Ionization energy anomalies in groups [edit]
Ionization energy values tend to subtract on going to heavier elements within a grouping[13] as shielding is provided by more electrons and overall, the valence shells experience a weaker allure from the nucleus, attributed to the larger covalent radius which increase on going down a grouping[32] Nonetheless, this isn't always the case. As one exception, in Grouping 10 palladium (
46 Pd
: 8.34 eV) has a college ionization energy than nickel (
28 Ni
: seven.64 eV), contrary to the general decrease for the elements from technetium
43 Tc
to xenon
54 Xe
. Such anomalies are summarized below:
- Group ane:
- Hydrogen's ionization energy is very high (at xiii.59844 eV), compared to the alkali metals. This is due to its single electron (and hence, very pocket-sized electron cloud), which is close to the nucleus. Likewise, since there aren't whatever other electrons that may cause shielding, that unmarried electron experiences the total internet positive charge of the nucleus.[33]
- Francium's ionization free energy is college than the precedent alkali metal, cesium. This is due to its (and radium's) minor ionic radii attributable to relativistic effects. Because of their big mass and size, this means that its electrons are traveling at extremely high speeds which results in the electrons coming closer to the nucleus than expected, and they are consequently harder to remove (college IE).[34]
- Group ii: Radium'south ionization energy which is higher than its ancestor alkaline world metal barium, like francium, is as well due to relativistic furnishings. The electrons, peculiarly the 1s electrons, feel very loftier effective nuclear charges. To avert falling into the nucleus, the 1s electrons must orbit at very high speeds, which causes the special relativistic corrections to be substantially higher than the approximate classical momenta. Past the doubtfulness principle, this causes a relativistic contraction of the 1s orbital (and other orbitals with electron density shut to the nucleus, especially ns and np orbitals). Hence this causes a cascade of electron changes which finally results in the outermost electron shells contracting and getting closer to the nucleus.
- Group 4:
- Hafnium's near similarity in IE than zirconium. The furnishings of the lanthanide wrinkle can all the same be felt afterwards the lanthanides.[thirty] It can exist seen through the former'south lesser diminutive radii (which contradicts the observed periodic tendency) at 159 pm[35] (empirical value) which differs from the latter's 155 pm.[36] [37] This in turn makes its ionization energies increase by eighteen±kJ/mol−1.
- Titanium'southward IE, which is lesser than both hafnium and zirconium. Hafnium'southward ionization energy is similar to zirconium due to lanthanide wrinkle. However, why zirconium's ionization energy is higher than the element preceding it remains shrouded; nosotros cannot rule atomic radii as in fact it is higher for zirconium and hafnium by 15 pm.[38] We likewise cannot rule the condensed ionization energy, as they're more or less the aforementioned ([Ar] 3d2 4s2 for titanium, whereas [Kr] 4d2 5sii for zirconium). Additionally, there are no half-filled nor fully filled orbitals we might compare. Hence, nosotros can only rule out zirconium's total electron configuration, which is 1s22stwo2phalf dozen3sii3psix 3d10 4s24phalf dozen4d25stwo.[39] The presence of a full 3d-cake sublevel is tantamount to a higher shielding efficiency compared to the 4d-cake elements (which are only 2 electrons).[a]
- Group 5: alike to Grouping iv, niobium and tantalum are analogous to each other, due to their electron configuration and to the lanthanide wrinkle affecting the latter element.[twoscore] Ipso facto, their significant ascent in IE compared to the foremost element in the group, vanadium, can exist attributed due to their full d-block electrons, in addition to their electron configuration. Another intriguing notion is niobium's half-filled 5s orbital; due to repulsion and exchange energy (in other words the "costs" for putting an electron in a low-energy sublevel to completely fill it instead of putting the electron in a high-energy one) overcoming the energy gap between s- and d-(or f) block electrons, the EC does not follow the Madelung dominion.
- Group six: like its forerunners groups 4 and five, group six likewise record high values when moving downward. Tungsten is in one case over again similar to molybdenum due to their electron configurations.[41] As well, information technology is also attributed to the full 3d-orbital in its electron configuration. Another reason is molybdenum's half filled 4d orbital due to electron pair energies violating the aufbau principle.
- Groups 7-12 6th period elements (rhenium, osmium, iridium, platinum, gold and mercury): All of these elements accept extremely high ionization energies than the chemical element preceding them in their corresponding groups. The essence of this is due to the lanthanide wrinkle's influence to post lanthanides, in improver to the relativistic stabilization of the 6s orbital.
- Group 13:
- Gallium's IE which is higher than aluminum. This is one time again due to d-orbitals, in addition to scandide contraction, providing weak shielding, and hence the constructive nuclear charges are augmented.
- Thallium'southward IE, due to poor shielding of 4f electrons[five] in addition to lanthanide contraction, causes it's IE to exist heightened in dissimilarity to its forerunner indium.
- Group xiv: Lead's unusually high ionization energy (
82 Pb
: vii.42 eV) is, alike to that of group 13'southward thallium, a upshot of the total 5d and 4f subshells. The lanthanide contraction and the inefficient screening of the nucleus by the 4f electrons results in slightly higher ionization energy for atomic number 82 than for tin (
50 Sn
: 7.34 eV).[42] [5]
Bohr model for hydrogen atom [edit]
The ionization energy of the hydrogen atom ( ) tin can be evaluated in the Bohr model,[43] which predicts that the atomic energy level has energy
RH is the Rydberg abiding for the hydrogen atom. For hydrogen in the footing land and then that the energy of the atom before ionization is simply
Later on ionization, the energy is goose egg for a motionless electron infinitely far from the proton, and so that the ionization free energy is
- . This agrees with the experimental value for the hydrogen atom.
Breakthrough-mechanical explanation [edit]
| | This section needs expansion with: more calculation formulas for ionization energies. You can assistance by calculation to it. (September 2020) |
Co-ordinate to the more complete theory of breakthrough mechanics, the location of an electron is all-time described as a probability distribution within an electron cloud, i.east. diminutive orbital.[44] [45] The energy can exist calculated by integrating over this cloud. The cloud'due south underlying mathematical representation is the wavefunction which is congenital from Slater determinants consisting of molecular spin orbitals.[46] These are related by Pauli'southward exclusion principle to the antisymmetrized products of the atomic or molecular orbitals.
There are two main ways ionization energy is calculated. In general, the computation for the Nth ionization energy requires calculating the energies of and electron systems. Calculating these energies exactly is not possible except for the simplest systems (i.e. hydrogen and hydrogen-like elements), primarily because of difficulties in integrating the electron correlation terms.[47] Therefore, approximation methods are routinely employed, with dissimilar methods varying in complexity (computational time) and accuracy compared to empirical information. This has become a well-studied problem and is routinely done in computational chemical science. The 2nd manner of calculating ionization energies is mainly used at the lowest level of approximation, where the ionization energy is provided by Koopmans' theorem, which involves the highest occupied molecular orbital or "Homo" and the lowest unoccupied molecular orbital or "LUMO", and states that the ionization energy of an atom or molecule is equal to the energy of the orbital from which the electron is ejected.[48] This means that the ionization energy is equal to the HOMO energy, whose formal equation is equal to: .[49]
Molecules: vertical and adiabatic ionization energy [edit]

Figure 1. Franck–Condon principle energy diagram. For ionization of a diatomic molecule, the simply nuclear coordinate is the bail length. The lower curve is the potential energy curve of the neutral molecule, and the upper curve is for the positive ion with a longer bond length. The blueish arrow is vertical ionization, here from the ground state of the molecule to the five=2 level of the ion.
Ionization of molecules oft leads to changes in molecular geometry, and 2 types of (first) ionization energy are defined – adiabatic and vertical.[50]
Adiabatic ionization free energy [edit]
The adiabatic ionization energy of a molecule is the minimum amount of energy required to remove an electron from a neutral molecule, i.e. the difference between the free energy of the vibrational ground state of the neutral species (v" = 0 level) and that of the positive ion (v' = 0). The specific equilibrium geometry of each species does not affect this value.
Vertical ionization free energy [edit]
Due to the possible changes in molecular geometry that may result from ionization, additional transitions may exist betwixt the vibrational ground state of the neutral species and vibrational excited states of the positive ion. In other words, ionization is accompanied by vibrational excitation. The intensity of such transitions is explained past the Franck–Condon principle, which predicts that the well-nigh probable and intense transition corresponds to the vibrationally excited state of the positive ion that has the aforementioned geometry as the neutral molecule. This transition is referred to as the "vertical" ionization energy since information technology is represented by a completely vertical line on a potential energy diagram (run across Figure).
For a diatomic molecule, the geometry is defined by the length of a single bond. The removal of an electron from a bonding molecular orbital weakens the bond and increases the bond length. In Figure 1, the lower potential energy curve is for the neutral molecule and the upper surface is for the positive ion. Both curves plot the potential energy as a function of bond length. The horizontal lines represent to vibrational levels with their associated vibrational moving ridge functions. Since the ion has a weaker bond, it will take a longer bond length. This consequence is represented by shifting the minimum of the potential energy curve to the correct of the neutral species. The adiabatic ionization is the diagonal transition to the vibrational basis state of the ion. Vertical ionization may involve vibrational excitation of the ionic state and therefore requires greater energy.
In many circumstances, the adiabatic ionization energy is often a more than interesting physical quantity since it describes the departure in free energy betwixt the two potential energy surfaces. However, due to experimental limitations, the adiabatic ionization energy is often difficult to decide, whereas the vertical disengagement energy is easily identifiable and measurable.
Analogs of ionization energy to other systems [edit]
While the term ionization energy is largely used merely for gas-phase atomic or molecular species, there are a number of analogous quantities that consider the amount of energy required to remove an electron from other physical systems.
Electron binding energy [edit]
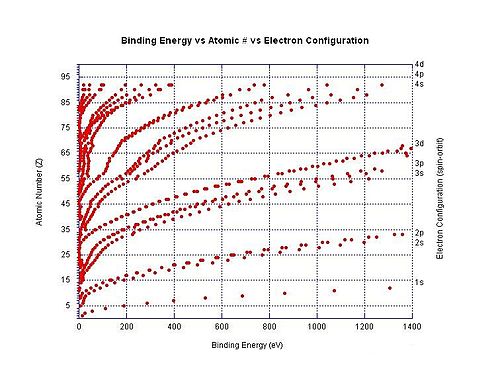
Bounden energies of specific atomic orbitals as a function of the atomic number. Because of the increasing number of protons, electrons occupying the same orbital are more than tightly bound in heavier elements.
Electron binding free energy is a generic term for the minimum energy needed to remove an electron from a particular electron shell for an cantlet or ion, due to these negatively charged electrons being held in identify by the electrostatic pull of the positively charged nucleus.[51] For example, the electron binding energy for removing a 3pthree/2 electron from the chloride ion is the minimum amount of energy required to remove an electron from the chlorine atom when it has a charge of -1. In this particular case, the electron bounden energy has the same magnitude as the electron affinity for the neutral chlorine atom. In another example, the electron binding energy refers to the minimum corporeality of free energy required to remove an electron from the dicarboxylate dianion −OtwoC(CHii)viiiCO −
two .
The graph to the right shows the binding energy for electrons in different shells in neutral atoms. The ionization energy is the lowest bounden free energy for a particular atom (although these are not all shown in the graph).
Solid surfaces: work function [edit]
Work part is the minimum amount of energy required to remove an electron from a solid surface, where the work function West for a given surface is divers by the difference[52]
where −e is the charge of an electron, ϕ is the electrostatic potential in the vacuum nearby the surface, and E F is the Fermi level (electrochemical potential of electrons) within the material.
Note [edit]
- ^ Notwithstanding, further inquiry is nevertheless needed to corroborate this mere inference.
See also [edit]
- Rydberg equation, a calculation that could determine the ionization energies of hydrogen and hydrogen-like elements. This is further elaborated through this site.
- Electron affinity, a closely related concept describing the energy released by adding an electron to a neutral atom or molecule.
- Lattice energy, a measure of the free energy released when ions are combined to brand a compound.
- Electronegativity is a number that shares some similarities with ionization energy.
- Koopmans' theorem, regarding the predicted ionization energies in Hartree–Fock theory.
- Ditungsten tetra(hpp) has the lowest recorded ionization energy for a stable compound.
- Bond-dissociation energy, the measure out of the strength of a chemical bail calculated through cleaving by homolysis giving ii radical fragments A and B and subsequent evaluation of the enthalpy change
- Bond energy, the average measure of a chemical bond'due south forcefulness, calculated through the amount of heat needed to pause all of the chemical bonds into individual atoms.
References [edit]
- ^ "Periodic Trends". Chemistry LibreTexts. 2013-10-02. Retrieved 2020-09-xiii .
- ^ a b c Miessler, Gary 50.; Tarr, Donald A. (1999). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 41. ISBN0-thirteen-841891-8.
- ^ "Ionization Energy". ChemWiki. Academy of California, Davis. 2013-x-02.
- ^ "Chapter ix: Quantum Mechanics". faculty.chem.queesu.ca. January 15, 2018. Retrieved October 31, 2020.
- ^ a b c d Lang, Peter F.; Smith, Barry C. (Baronial 2003). "Ionization Energies of Atoms and Atomic Ions". Journal of Chemical Pedagogy. 80 (viii): 938. Bibcode:2003JChEd..80..938L. doi:10.1021/ed080p938.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gilded Book") (1997). Online corrected version: (2006–) "ionization potential". doi:x.1351/goldbook.I03208
- ^ Cotton wool, F. Albert; Wilkinson, Geoffrey (1988). Advanced Inorganic Chemical science (5th ed.). John Wiley. p. 1381. ISBN0-471-84997-9.
- ^ Mahan, Bruce H. (1962). "Ionization Energy". Higher of Chemistry, Academy of California Berkeley. Retrieved 2020-09-thirteen .
- ^ "Monatomic Gas - an overview | ScienceDirect Topics". www.sciencedirect.com . Retrieved 2022-01-08 .
- ^ a b Rock, East.G. (December nineteen, 2020v). "Atomic Structure : Periodic Trends". Department of Chemistry. chem.tamu.edu. 400 Bizzell St, College Station, TX 77843, Texas, U.s.: Texas A&One thousand University. Retrieved December xix, 2020.
{{cite web}}: CS1 maint: location (link) - ^ "Anomalous trends in ionization free energy". Chemical science Stack Exchange . Retrieved 2020-09-20 .
- ^ Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geoffrey (2002). General Chemistry (eighth ed.). Prentice Hall. p. 370. ISBN0-13-014329-4.
- ^ a b Grandinetti, Philip J. (September viii, 2019). "Ionization Free energy Trends | Grandinetti Group". www.grandinetti.org . Retrieved 2020-09-13 .
- ^ Kent, Mr. "Commencement Ionization Energy". kentchemistry.com. KentChemistry. Retrieved December 6, 2020.
...The add-on of the second electron into an already occupied orbital introduces repulsion between the electrons, thus information technology is easier to remove. that is why there is a dip in the ionization free energy.
- ^ "Group IA". chemed.chem.purdue.edu . Retrieved 2020-09-20 .
- ^ "Brine Metals". hyperphysics.phy-astr.gsu.edu . Retrieved 2020-09-13 .
- ^ "The Alkali Metals | Introduction to Chemical science". courses.lumenlearning.com . Retrieved 2020-09-13 .
- ^ "Chemical elements listed past ionization energy". lenntech.com. Lenntech BV. 2018. Retrieved December vi, 2020.
The elements of the periodic table sorted by ionization free energy click on whatever element's name for further data on chemical properties, environmental information or wellness effects. This listing contains the 118 elements of chemistry.
- ^ Boudreaux, K.A. (Baronial 13, 2020) [July 26, 2006]. "The Parts of the Periodic Table". Department of Chemistry and Biochemistry. angelo.edu/faculty/kboudrea/. 2601 Due west. Avenue N, San Angelo, TX 76909, Texas: Angelo State University. Retrieved December nineteen, 2020 – via angelo.edu.
{{cite web}}: CS1 maint: location (link) - ^ "xviii.ten: The Grouping 6A Elements". Chemistry LibreTexts. 2014-07-02. Retrieved 2020-09-xx .
- ^ "Covalent Radius for all the elements in the Periodic Table". periodictable.com . Retrieved 2020-09-13 .
- ^ Sikander (December 5, 2015). "Why is ionisation free energy of bismuth lower than pb?". chemistry.stackexchange.com. Chemistry Stack Substitution. Retrieved December five, 2020.
Why is ionisation enthalpy of Bismuth less than that of Pb for information technology just comes afterward the latter in periodic table?
- ^ Ball, Philip (April 21, 2017). "The group 3 dilemma". chemistryworld.com. Burlington Business firm, Piccadilly, London: Chemistry Earth. Retrieved December 18, 2020 – via Royal Society of Chemical science.
- ^ Yirka, Bob (Apr 9, 2015). "Measurement of first ionization potential of lawrencium reignites debate over periodic table". General Physics. phys.org. Phys Org. Phys.org. Retrieved Dec 13, 2020.
Lawrencium, at this fourth dimension, appears to have a dumb-bong shape. These new findings create alien views on where the element should be placed on the table and has reignited debate on the way the table is structured in general.
- ^ Scerri, Eric R.; Parsons, William (March 2017). "What elements belong in grouping iii of the periodic tabular array?". world wide web.ionicviper.org. Ionic Viper. Retrieved December 7, 2020.
The question of precisely which elements should be placed in group 3 of the periodic table has been debated from time to time with plain no resolution up to this indicate.
- ^ Sato, T. Chiliad.; Asai, K.; Borschevsky, A.; Stora, T.; Sato, Northward.; Kaneya, Y.; Tsukada, K.; Düllmann, Ch E.; Eberhardt, G.; Eliav, Due east.; Ichikawa, Due south.; Kaldor, U.; Kratz, J. V.; Miyashita, S.; Nagame, Y.; Ooe, K.; Osa, A.; Renisch, D.; Runke, J.; Schädel, M.; Thörle-Pospiech, P.; Toyoshima, A.; Trautmann, N. (April 2015). "Measurement of the first ionization potential of lawrencium, element 103". Nature. 520 (7546): 209–211. Bibcode:2015Natur.520..209S. doi:ten.1038/nature14342. PMID 25855457. S2CID 4384213.
- ^ Singh, Jasvinder (1999). "Inert Gases". Sterling Lexicon of Physics. Sterling Publishers Pvt. Ltd. p. 122. ISBN978-81-7359-124-2.
- ^ "Vanadium, Niobium and Tantalum". Chemistry of the Elements. 1997. pp. 976–1001. doi:10.1016/B978-0-7506-3365-9.50028-6. ISBN978-0-7506-3365-9.
- ^ Housecroft, C.E.; Sharpe, A.G. (Nov 1, 1993). Inorganic Chemistry (eBook). Inorganic Chemical science. Vol. 3 (15th ed.). Switzerland: Pearson Prentice-Hall. pp. 536, 649, 743. doi:ten.1021/ed070pA304.1. ISBN978-0-273-74275-3. Archived from the original on Dec 16, 2015. Retrieved Dec 14, 2020.
- ^ a b Cotton wool, F. Albert; Wilkinson, Geoffrey (1988), Advanced Inorganic Chemistry (fifth ed.), New York: Wiley-Interscience, pp. 776, 955, ISBN0-471-84997-ix
- ^ Billo, Due east. J. (1985). "Modern Inorganic Chemistry (Jolly, William 50.)". Periodical of Chemical Didactics. 62 (4): A137. Bibcode:1985JChEd..62..137B. doi:10.1021/ed062pA137.1.
- ^ "Patterns and trends in the periodic table - Periodicity - Higher Chemistry Revision". BBC Bitesize . Retrieved 2020-09-20 .
- ^ "Ionization Energies". Chemistry LibreTexts. 2013-10-03. Retrieved 2020-09-20 .
- ^ "IYPT 2019 Elements 087: Francium: Not the most reactive Group 1 element". Compound Interest. 2019-11-06. Retrieved 2020-09-20 .
- ^ "Hafnium". gordonengland.co.uk. Gordon England. 2020. Retrieved December 7, 2020.
...Atomic Radius 159 pm...
- ^ "Zirconium (Element) - Atomic Radius". pubchem.ncbi.nlm.nih.gov. PubChem. Retrieved December 8, 2020.
155 pm (Empirical)
- ^ Slater, J. C. (xv November 1964). "Diminutive Radii in Crystals". The Journal of Chemic Physics. 41 (10): 3199–3204. Bibcode:1964JChPh..41.3199S. doi:x.1063/1.1725697.
- ^ "WebElements Periodic Table » Titanium » radii of atoms and ions". www.webelements.com . Retrieved 2020-09-xx .
- ^ Straka, J. "Periodic Tabular array of the Elements: Zirconium - Electronic configuration". www.tabulka.cz . Retrieved 2020-09-xx .
- ^ "Tantalum | chemical element". Encyclopedia Britannica . Retrieved 2020-09-twenty .
- ^ Langård, Sverre (2015). "Chromium, Molybdenum, and Tungsten". Patty's Toxicology. doi:ten.1002/0471435139.tox038. ISBN978-0-471-12547-one.
- ^ "The Group 14 elements". Chemistry Nexus. 2015-12-02. Retrieved 2020-09-13 .
- ^ Bohr, Northward. (July 1913). "I. On the constitution of atoms and molecules". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 26 (151): ane–25. doi:x.1080/14786441308634955.
- ^ "Orbitals, Electron Clouds, Probabilities, and Energies". chem.libretexts.org. UC Davis ChemWiki. May 23, 2019. Retrieved Nov 2, 2020.
- ^ "Quantum numbers and orbitals- The breakthrough mechanical model of the atom". Khan Academy . Retrieved November ii, 2020.
- ^ Levine 1991, p. 315: "In the Hartree-Fock approximation, the wave function of an atom (or molecule) is a Slater determinant or a linear combination of a few Slater determinants"
- ^ Levine 1991, pp. 290–291.
- ^ Levine 1991, p. 475.
- ^ "Groundwork Reading for Ionization Energy". shodor.org. The Shodor Education Foundation, Inc. 2000. Retrieved November 15, 2020.
... The second method is called Koopman'southward Theory. This method involves the Homo.
- ^ "The difference between a vertical ionization energy and adiabatic ionization energy". Computational Chemistry Comparison and Benchmark Database. National Constitute of Standards and Technology.
- ^ White potato, Andrew; Wong, Monica (2019). "Electron binding energy". radiopaedia.org. Radiopaedia. Retrieved Dec seven, 2020.
The electron bounden energy is the minimum energy that is required to remove an electron from an cantlet
- ^ Kittel, Charles (January 1, 1996) [1953]. "half-dozen". In Zainab, R.; Du, D.; Tanner, B.K.; Chambers, R.G. (eds.). Introduction to Solid Land Physics. Physics Today. Vol. 7. New York, United states of america: John Wiley & Sons, Inc. (published 1995). pp. 18–19. Bibcode:1969Natur.224..983C. doi:10.1063/1.3061720. ISBN978-0-471-11181-8. LCCN 95-018445. OCLC 263625446. S2CID 121571376. Archived from the original on Jan 13, 2017. Retrieved December eighteen, 2020. [ page needed ]
Sources [edit]
- Levine, Ira Due north. (1991). Breakthrough Chemistry. Prentice Hall. ISBN978-0-205-12770-2.
Source: https://en.wikipedia.org/wiki/Ionization_energy










0 Response to "What Is the Minimum Energy Required to Ionize a Hydrogen Atom in the N=3"
Publicar un comentario